Dietary supplements are regulated by the us. According to the fda regulations most nutraceuticals would be categorized as dietary supplements.
fda regulations for vitamins and supplements is important information accompanied by photo and HD pictures sourced from all websites in the world. Download this image for free in High-Definition resolution the choice "download button" below. If you do not find the exact resolution you are looking for, then go for a native or higher resolution.
Don't forget to bookmark fda regulations for vitamins and supplements using Ctrl + D (PC) or Command + D (macos). If you are using mobile phone, you could also use menu drawer from browser. Whether it's Windows, Mac, iOs or Android, you will be able to download the images using download button.
The law defines dietary supplements in part as products taken by mouth that contain a dietary ingredient dietary ingredients include vitamins minerals amino acids and herbs or botanicals as.

Fda regulations for vitamins and supplements. 1 as a result some unscrupulous manufacturers and marketers took advantage of the situation to make outlandish claims for vitamins and other dietary supplements. All prescription and non prescription drugs are regulated in the united states by the food and drug administration fda. It is the manufacturer and distributors of dietary supplements which are responsible for checking and making sure that their products are safe to use when the products are being marketed.
For the most up to date version of cfr title 21 go to the electronic code of federal regulations ecfr. Which put them under different regulations than drugs. Fda is not authorized to check dietary supplements and vitamins for safety before they are in the marketplace being used by consumers.
Fda regulates both finished dietary supplement products and dietary ingredients. Vitamins the daily values are the amounts of nutrients recommended per day for americans 4 years of age or older. 11 2019 healthday news the us.
Fda regulation of drugs versus dietary supplements. Food and drug administration fda which is given authority to regulate the industry through the dietary supplement health education act dshea. The fda established regulations in 1941 to govern labeling of vitamins establishing a minimum daily requirement for each vitamin but the agency did not restrict the amount of a vitamin allowed in supplements at that time.
Dshea is a federal statute passed in 1994 that defines what dietary supplements are and includes guidelines on how they should be sold and regulated. Fda regulates dietary supplements under a different set of regulations than those covering conventional foods and. One of the.
These are extracts concentrates or combinations of vitamins minerals botanicals herbs or dietary substances for use by man to supplement the diet by increasing the total dietary intake when is a nutraceutical a drug. Food and drug administration plans to strengthen regulation of dietary supplements such as vitamins minerals and herbs the agency announced. Fda vitamins and minerals chart author.
The information on this page is current as of april 1 2019. Fda regulation of drugs versus dietary supplements. Value biotin energy storage protein carbohydrate and fat.
Where is it found.
Fda Announces Final Regulations On Nutrition And Supplement

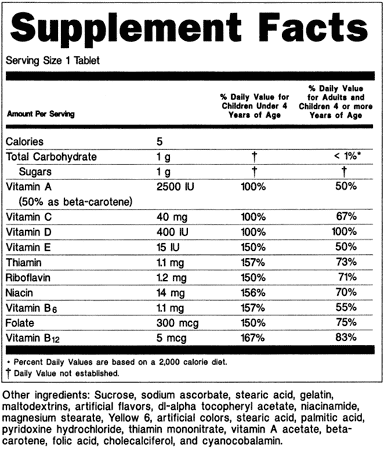
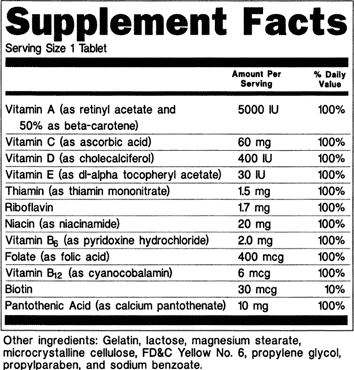
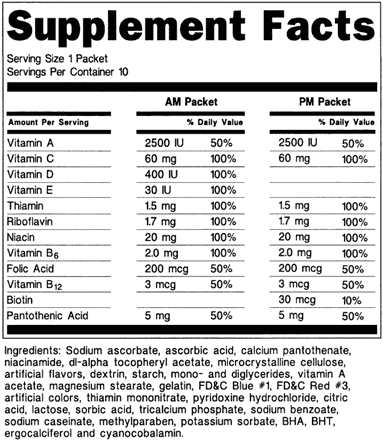
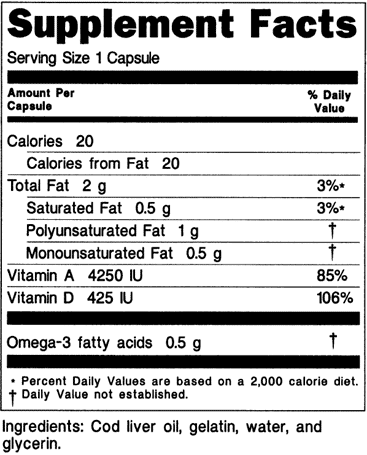
 Fda Advisory No 2019 187 Lifting The Advisory On The
Fda Advisory No 2019 187 Lifting The Advisory On The
 Do Fda Approved Vitamins And Supplements Exist
Do Fda Approved Vitamins And Supplements Exist
 Vitamins And Dietary Supplements Qualitycustomessays Com
Vitamins And Dietary Supplements Qualitycustomessays Com
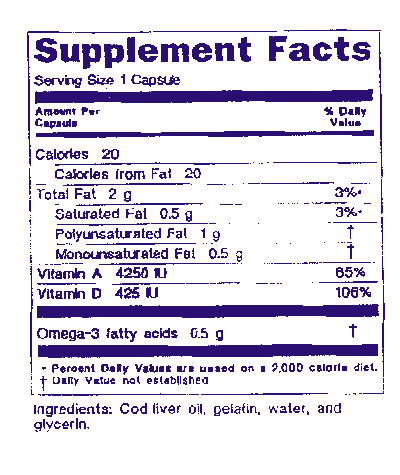 Small Entity Compliance Guide Statement Of Identity
Small Entity Compliance Guide Statement Of Identity

